- Blog
13/01/2026

Were you impressed by the precision of the nurse who took your blood sample? Now imagine the expertise required to do the same… on a mouse!
In many research fields, blood sampling is necessary to measure the levels of certain molecules and assess hormonal, immune, and hematological parameters, among others.
The most commonly used species for scientific purposes is the mouse (see Gircor infographic), a small mammal weighing about 25 g on average, with a total blood volume of 1.5 ml. The NC3Rs guidelines recommend a maximum blood volume of 10% of total blood volume for a single sampling, and up to 15% total for repeated sampling over a 28-day period. In humans, these limits correspond to blood donations: for a 70 kg individual with 70 ml of blood per kg, a total blood donation is 480 ml, which corresponds to 9.8% of the total blood volume (very close to the 10% recommended for a single sampling in mice.
Sometimes, small amounts of blood are sufficient for the necessary analyses. Pharmacokinetic and pharmacodynamic studies require (may be repeated) samples of less than 50 µl of blood to measure concentrations by LC-MS (Liquid Chromatography – Mass Spectrometry), a method that allows precise identification and/or quantification of many substances.
It is notably the improvement of assay protocols (requiring less blood) that has enabled repeated blood sampling from a single animal. This helps reduce the number of animals needed for a study (Leblanc et al., 2018; Reddy et al., 2012).
In these situations, several sampling routes can be used, for example:
Figure 1: Description of the blood sampling method at the mandibular vein in the mouse.
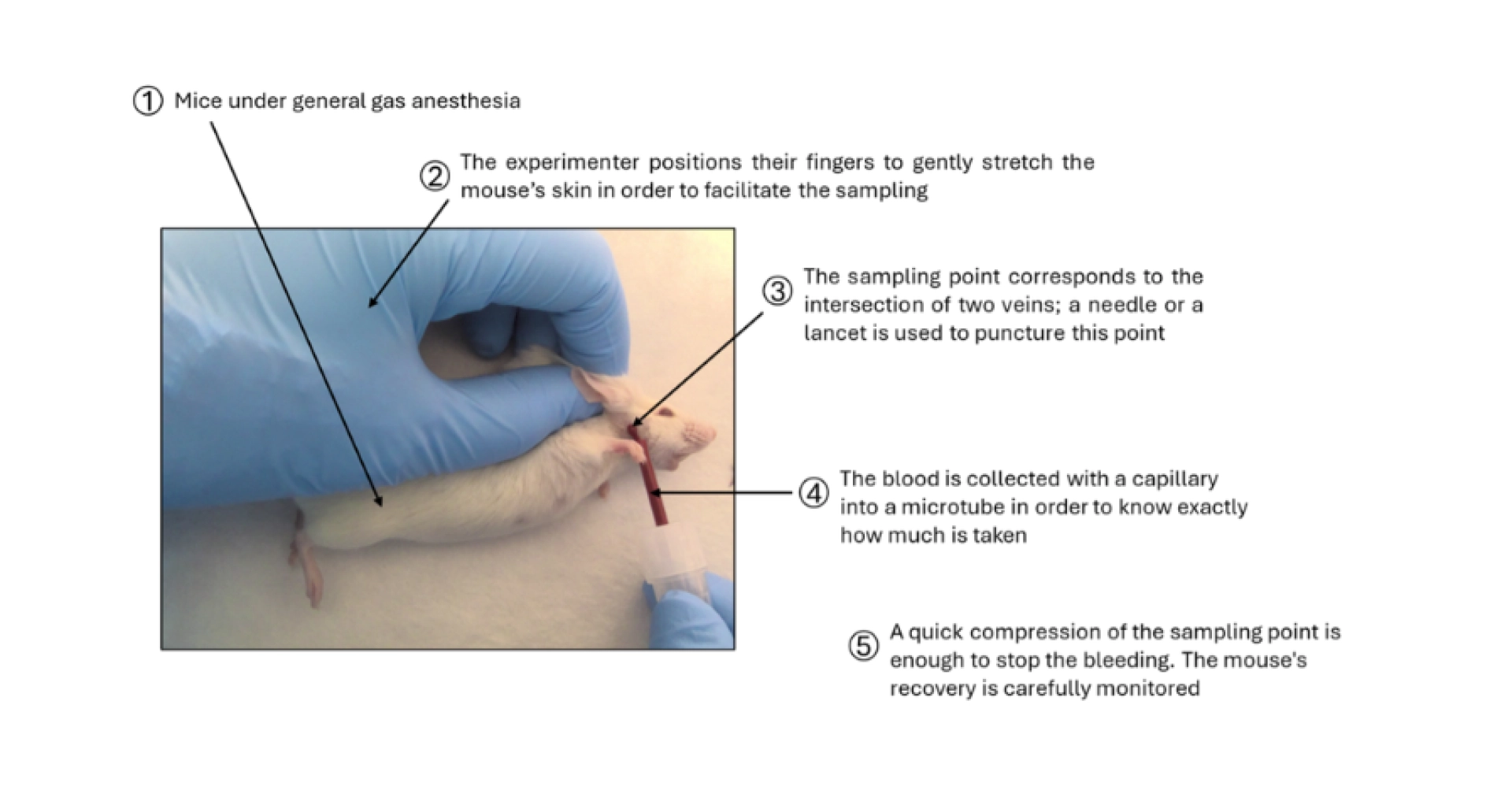
Figure 2: Description of the blood sampling method from the saphenous vein in the mouse
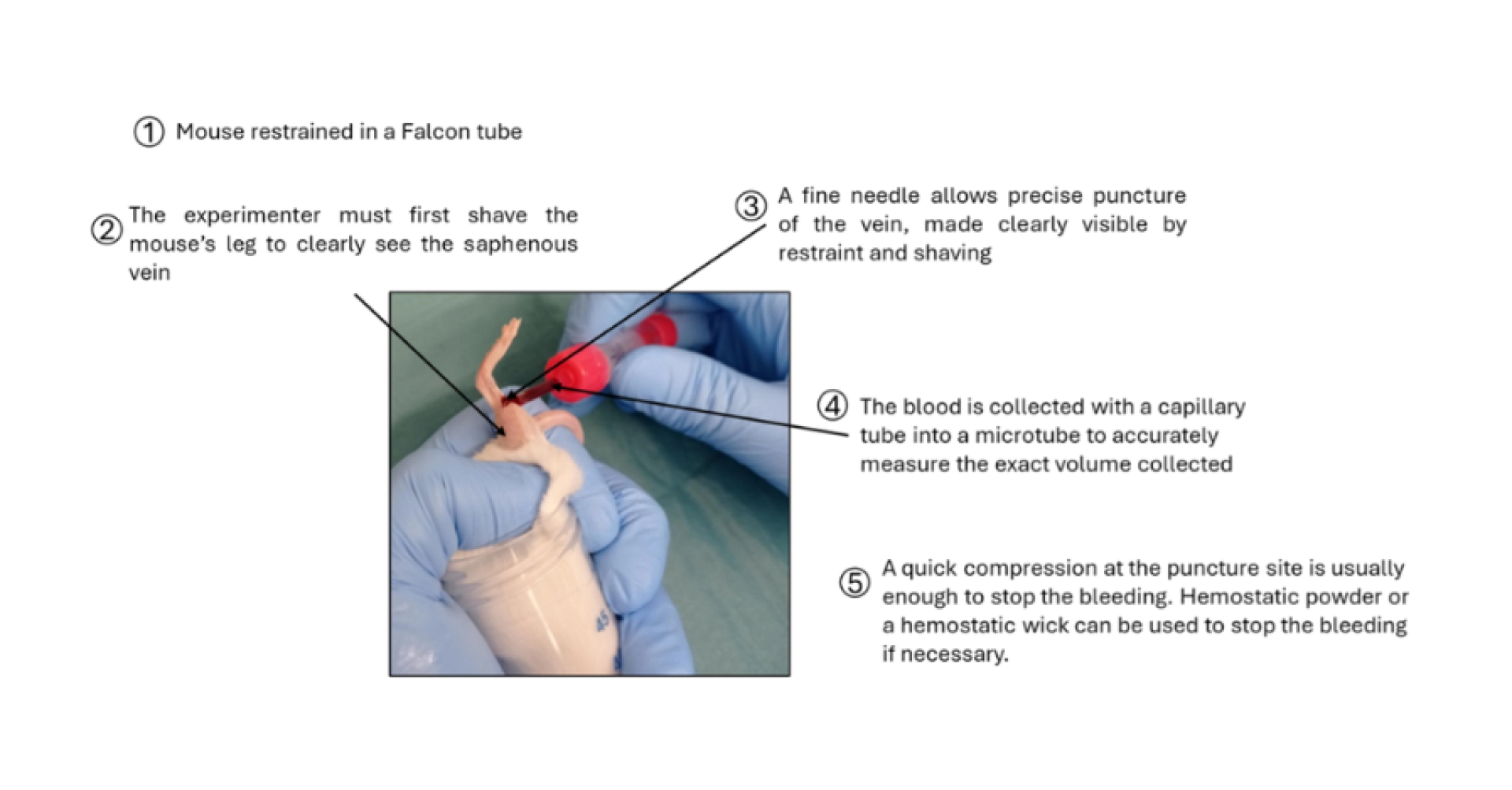
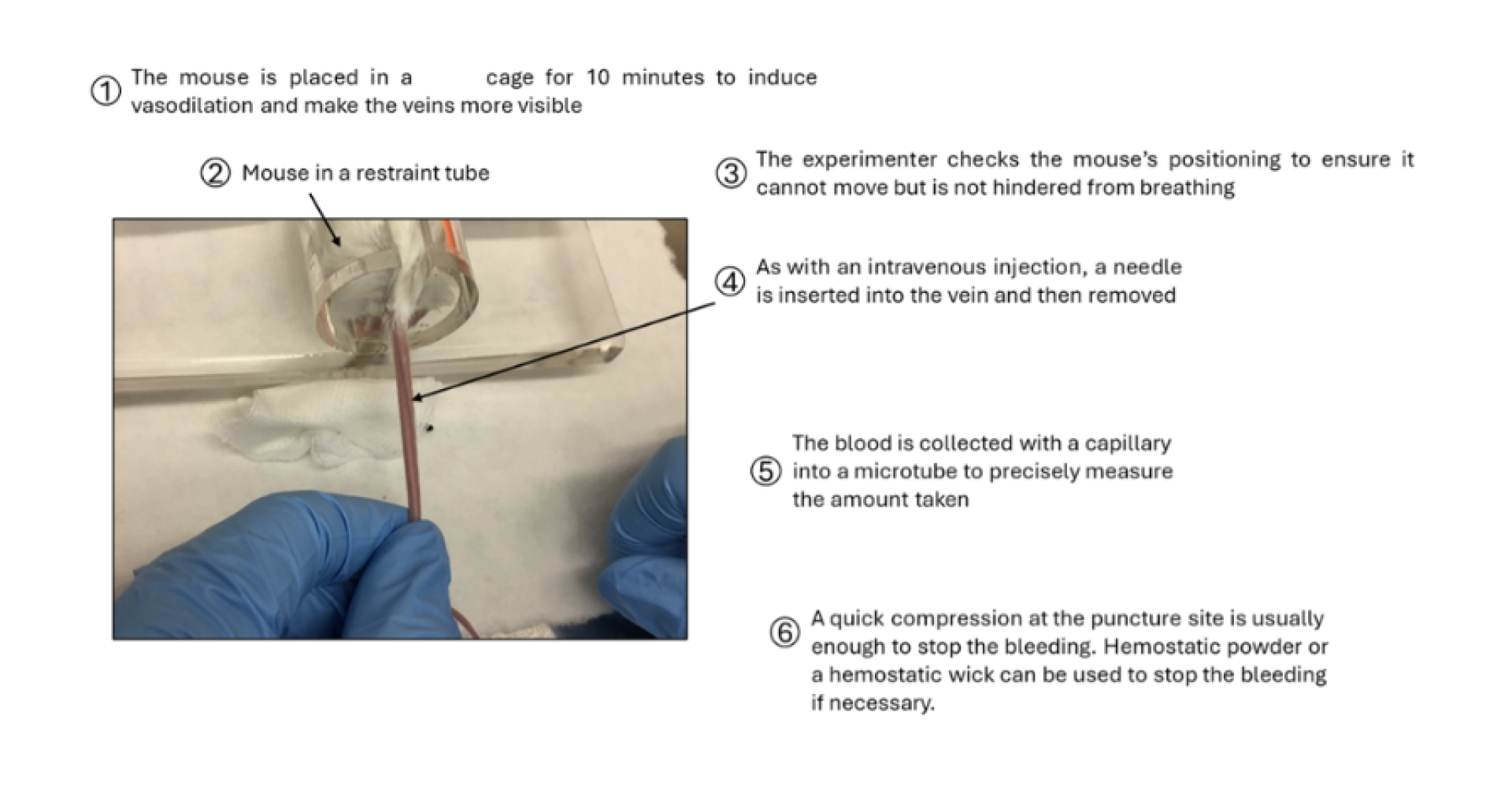
Figure 3: Description of the blood sampling method from the caudal vein in the mouse.
In all cases, the mouse is never returned to its cage until the bleeding has completely stopped.
Sometimes, especially when multiple analyses need to be performed from a single sample, larger volumes of blood are required (more than 200 µl). Beyond 10% of the total blood volume, the animal is at risk of anemia resulting from the blood collection. In such cases, it is recomended to perform the procedure under general anesthesia as a terminal procedure (the animal is euthanized at the end of the procedure, without awakening).
In this situation, several sampling routes can be used, for example:
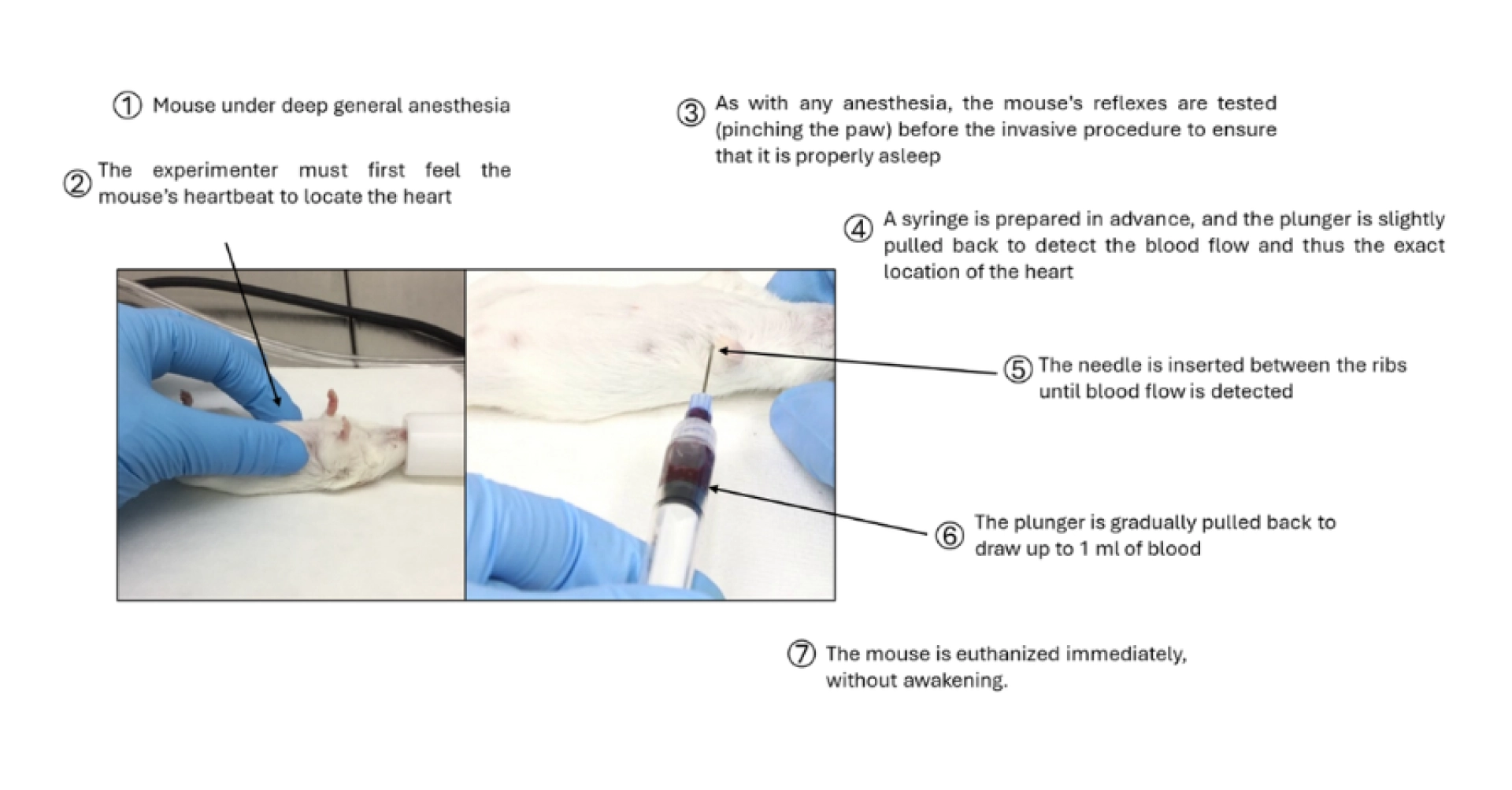
Figure 4: Description of the blood sampling method from the heart in the mouse.
Analgesia must be used if tissues are incised. For example, intracardiac sampling can be performed without incision (if the technicians are sufficiently trained); however, it is possible to incise the skin of the anesthetized animal for greater precision. In this case, appropriate analgesia is necessary.
There are therefore many blood sampling routes in mice. They must be carefully selected, first
based on scientific criteria (how much blood is needed, does the blood need to be perfectly
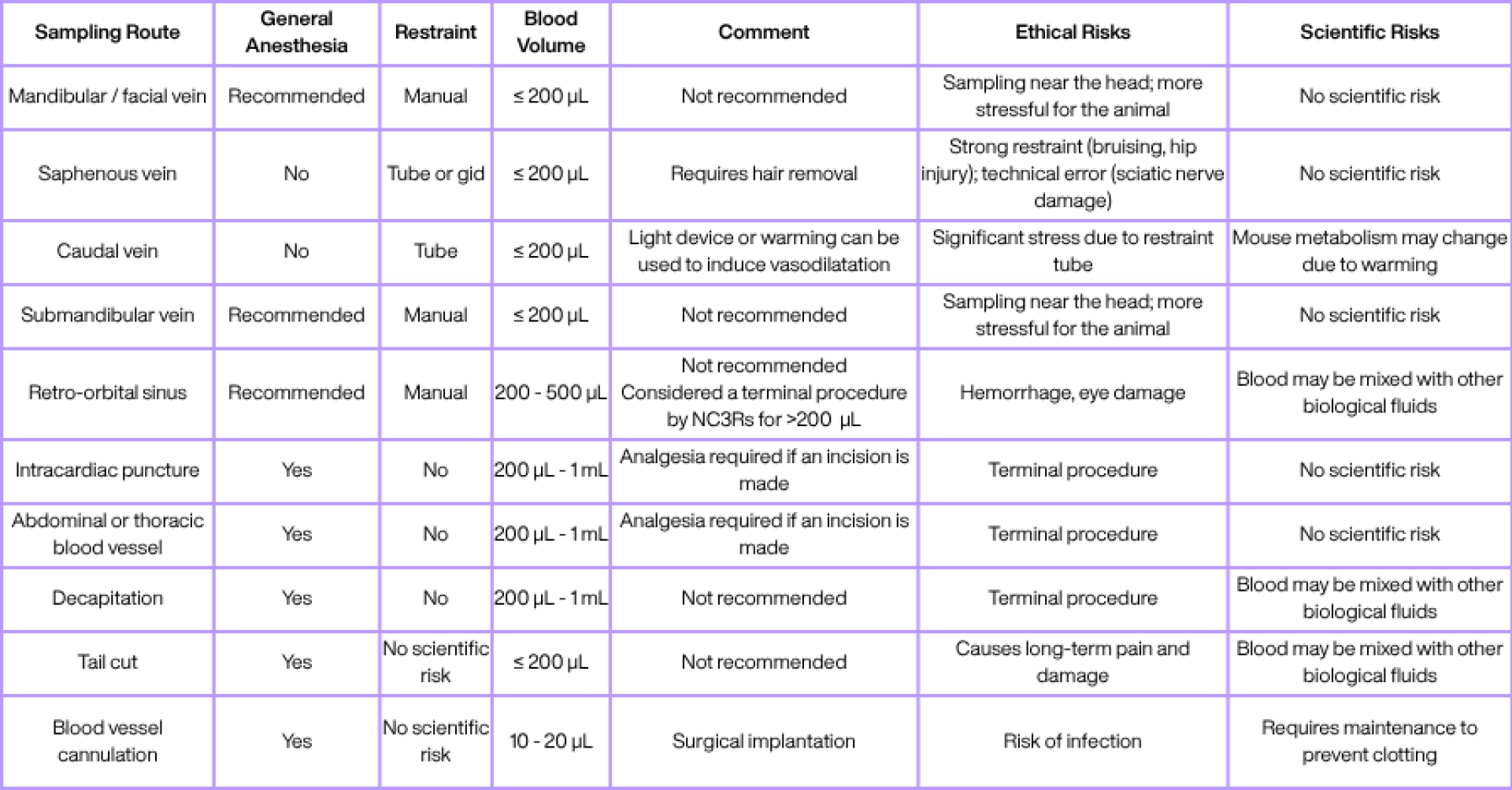
“pure,” etc.), and then based on ethical criteria (which method is least dangerous, painful, or stressful for the animal). Below, Tableau 1 presents a non-exhaustive list of blood sampling methods that can be used in mice (sources include the NC3Rs, FC3Rs, and studies by Diehl et al., 2001; Meyer et al., 2020; Whittaker & Barker, 2020).
The prioritization between scientific and ethical choices is simply explained by the fact that if ethical choice were prioritized alone, animals would no longer be used for scientific purposes.
After a significant blood collection (between 150 and 200 µl), in case of signs of dehydration (delayed skin tenting), and to help restore blood volume and prevent dehydration (for example, during a surgical procedure), rehydration may be necessary.
This is done using warmed physiological saline (between 20 and 37°C, depending on the volume) administered either intraperitoneally for rapid treatment (quick rehydration but a moderately invasive and less precise route) or subcutaneously for prevention (slower absorption but less invasive and lower risk) (see the blog “How to Administer a Substance to a Mouse”).
Tableau 1: Non-exhaustive list of blood sampling routes and their characteristics. The column regarding anesthesia does not reflect an ethical consideration, but a practical one: it indicates whether the sampling route can technically be performed without anesthesia.
Diehl, K.-H., Hull, R., Morton, D., Pfister, R., Rabemampianina, Y., Smith, D., Vidal, J.-M., & Van De Vorstenbosch, C. (2001). A Good Practice Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes. In JOURNAL OF APPLIED TOXICOLOGY J. Appl. Toxicol (Vol. 21).
Leblanc, A. F., Huang, K. M., Uddin, M. E., Anderson, J. T., Chen, M., & Hu, S. (2018). Murine Pharmacokinetic Studies. Bio-Protocol, 8(20), e3056. https://doi.org/10.21769/BIOPROTOC.3056
Meyer, N., Kröger, M., Thümmler, J., Tietze, L., Palme, R., & Touma, C. (2020). Impact of three commonly used blood sampling techniques on the welfare of laboratory mice: Taking the animal’s perspective. PLoS ONE, 15(9 september). https://doi.org/10.1371/journal.pone.0238895
Reddy, J., Madishetti, S., & Vachaspati, P. R. (2012). Fast mouse PK (Fast PK): A rapid screening method to increase pharmacokinetic throughput in pre-clinical drug discovery. European Journal of Pharmaceutical Sciences, 47(2), 444–450. https://doi.org/10.1016/J.EJPS.2012.07.001
Whittaker, A. L., & Barker, T. H. (2020). The impact of common recovery blood sampling methods, in mice (Mus musculus), on well-being and sample quality: A systematic review. In Animals (Vol. 10, Issue 6, pp. 1–32). MDPI AG. https://doi.org/10.3390/ani10060989