- Blog
13/01/2026

Mice are the most commonly used species in scientific research. These small rodents play a crucial role in drug development. During the preclinical phase, diseases are induced in mice to administer drug candidates and assess both their therapeutic effects and potential side effects. Whether to induce diseases or to test treatments, substances must be administered to mice.
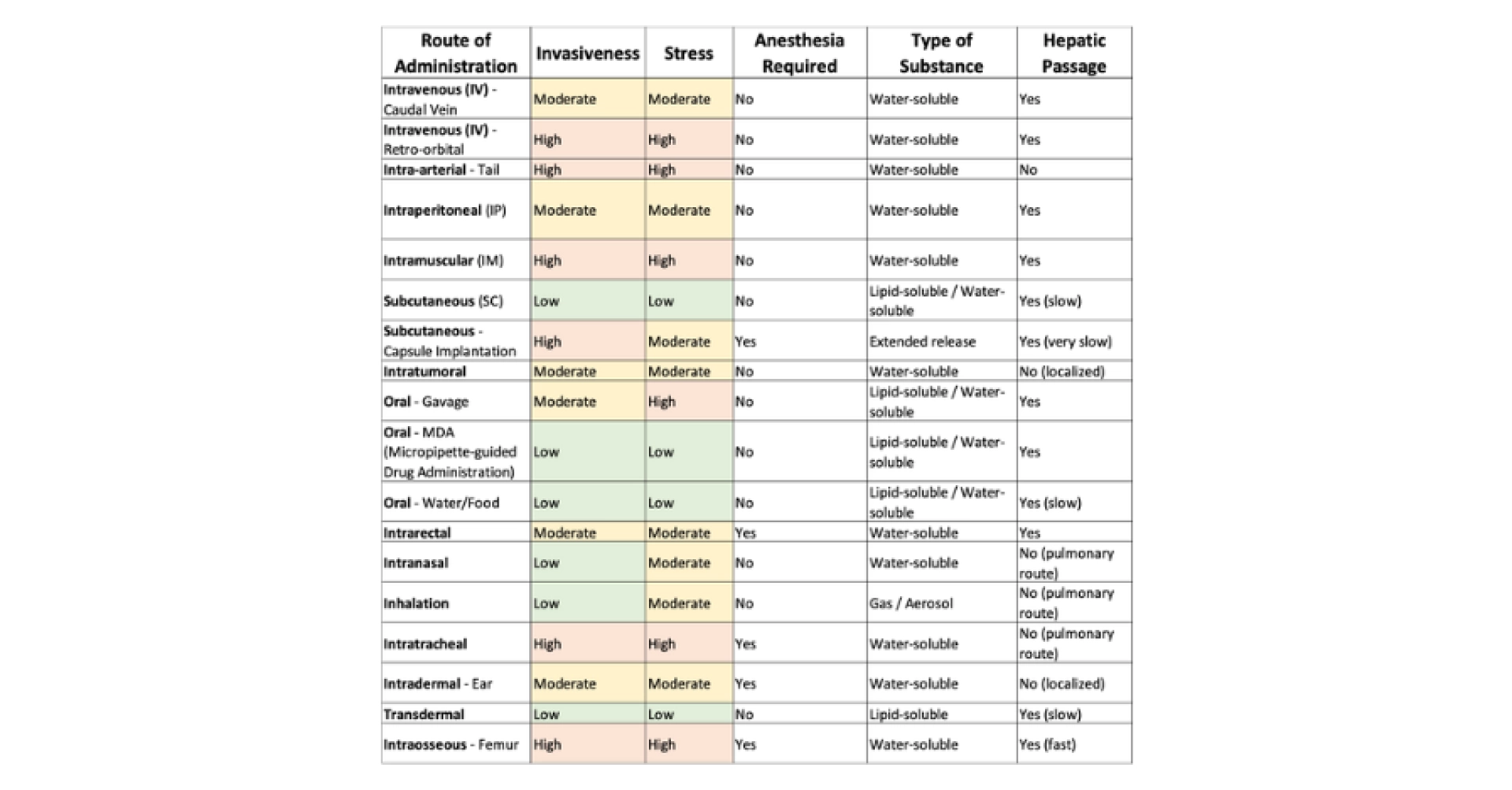
There are multiple administration routes, and their selection must be made carefully. The first priority is to consider scientific criteria, such as the appropriate diluent, the required dosage, and the intended route for the final drug. Once the most suitable scientific approach has been determined, ethical considerations come into play. The selected method should minimize harm, pain, and stress for the animal as much as possible. Table 1 below provides a non-exhaustive list of administration routes that can be used in mice. The sources referenced include NC3R, FC3R, Chapter 32 of The laboratory mouse (Hedrich, 2004) and (Turner et al., 2011). Scientific considerations take priority over ethical ones for a simple reason: if ethical concerns were the primary factor, animal research would no longer be conducted.
At TransCure bioServices, we continuously develop new procedures to expand the range of administration routes available, ensuring that we always select the most scientifically and ethically appropriate option. We prioritize the well-being of both the animals and our technicians by choosing methods with the least psychological impact. For this reason, retro-orbital administration is rarely used and only when a strong scientific justification demonstrates that it is the most suitable option.
Table 1: Non-exhaustive list of administration routes and their characteristics. The column related to anesthesia does not reflect an ethical consideration, but solely a practical one: it indicates whether the route of administration can be technically performed without anesthesia.
When a substance needs to be administered, it can serve different objectives:
• Are we aiming for a local or general effect? For example, local anesthesia would use a subcutaneous or intramuscular route, while general anesthesia would typically use an intravenous or inhalation route.
• Should the effect be rapid or long-term? For example, oral treatment can be administered via gavage or MDA (Micropipette-guided Drug Administration) for a rapid effect, or in drinking water for a long-term effect. A subcutaneous injection provides rapid diffusion, while using a capsule allows for slow diffusion.
• Has the formulation of the future medication been defined? For example, vaccines are generally administered via the intramuscular route; the medication is planned as a pill (oral route) or a suppository (intrarectal route).
The administration of a substance can also be subject to scientific constraints:
• Does the substance need to be administered in a large volume, or can it be concentrated? For example, intramuscular and intratumoral routes do not allow the administration of large volumes, unlike subcutaneous and intravenous routes (Diehl et al., 2001).
• Is the administered product viscous or liquid? For example, a viscous product cannot be administered intravenously, whereas the oral route (gavage or MDA) allows this.
The article by Morton and colleagues presents a checklist in Table 1 to anticipate potential challenges in a study (scientific objective; route of administration; characteristics of the substance to be administered; characteristics of the animals; etc.) (Morton et al., 2001).
If scientific objectives and constraints allow for the use of multiple routes of administration, the decision may be based on the pain and stress caused, as well as the potential danger to the animal.
Pain based on the route of administration:
Some routes of administration are more painful than others. The most painful routes are not necessarily the most invasive (in terms of tissue damage). Gavage and intratracheal routes are not invasive but irritate the esophagus and trachea. The intramuscular route is more painful than other invasive routes (such as the subcutaneous route).
Local analgesics can be used to limit pain in certain cases. Intratumoral administration is painful, and a local analgesic can be applied via the transdermal route to reduce pain.
Stress based on the route of administration:
Restraint, which may be more or less strict and for varying lengths of time, may be necessary for certain routes of administration. The location of the administration can be more or less stressful for the animal.
From head to feet / from ears to tail:
Manipulations that require an invasive procedure on or around the head of mice are considered more stressful than those targeting parts of the body farther from the head. This is particularly supported by studies comparing blood sampling (NC3R blood Sampling, (Whittaker & Barker, 2020)). This consideration also applies to intravenous routes, where multiple sites are possible, such as retro-orbital and tail vein. According to this principle, it is preferable to use the tail vein.
Freedom of movement:
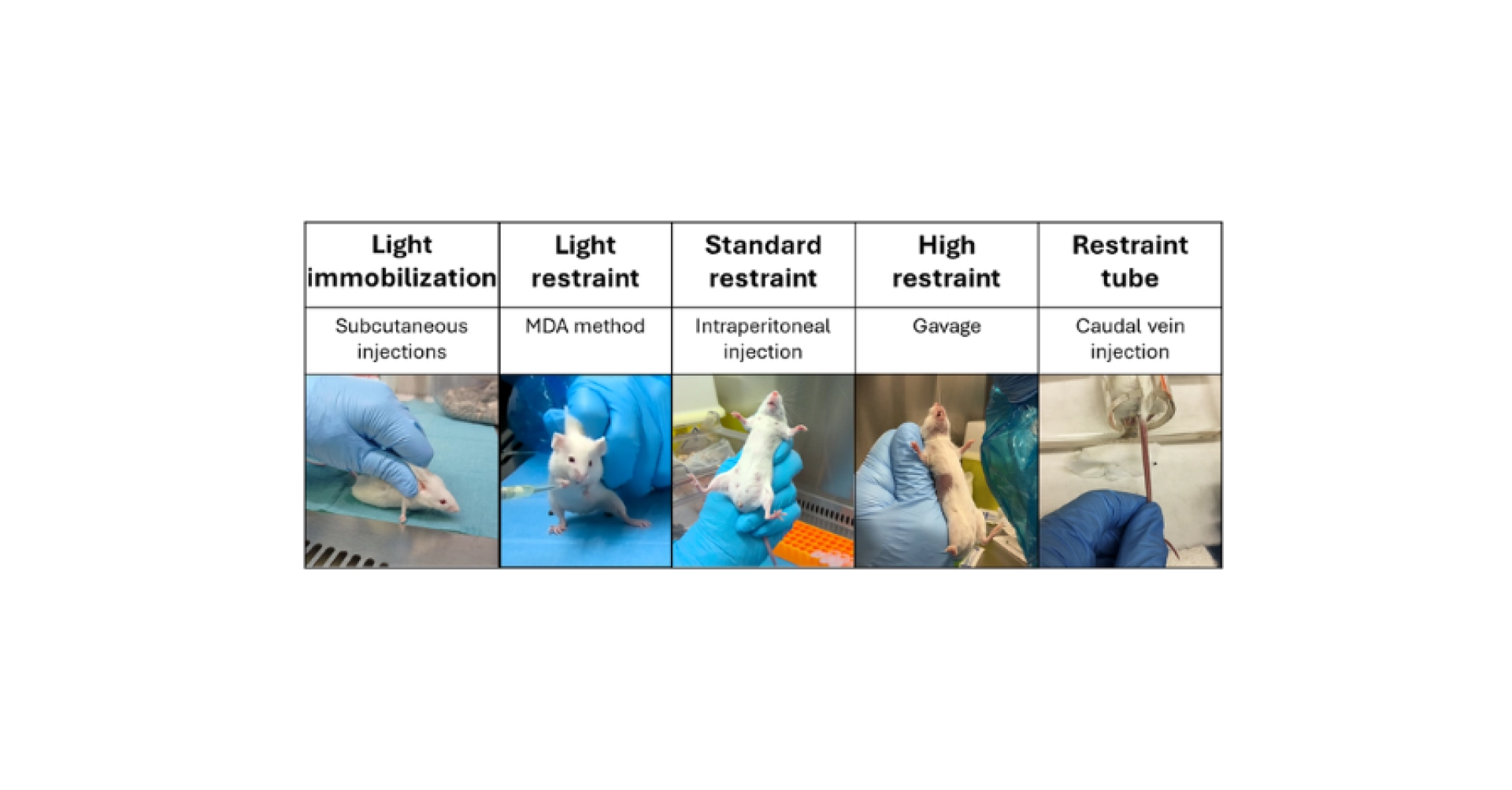
To administer a substance, animals may sometimes need to be restricted in their movements with a wide variety of constraints (Hedrich, 2004) (see Figure 1):
• No restraint: Administration via drinking water or directly in food does not require any restraint; the animal is free to move within its cage and drink or eat when it wishes.
• Restriction of movement space: Solid food can be used to administer substances (in gel pellets, jam, chocolate, etc.). To ensure each individual consumes its share, separations can be used in cages, or animals can be isolated while they eat (Huang-Brown & Guhad, 2002; Lax et al., 1983; Martins et al., 2022; Teixeira-Santos et al., 2021).
• Light immobilization: Subcutaneous injections between the shoulder blades can be administered by holding the mouse against the work surface.
• Light restraint: The MDA method can work without any restraint under certain conditions, but light handling (holding the mouse by the skin on its back keeping it on its hind legs without lifting it completely) allows this method to be used in more stressful environments (such as under a PSM, for example).
• Standard restraint: Intraperitoneal, subcutaneous injections at the flank, and intramuscular injections require restraint of the mouse, which allows the handler to bring the mouse close to their face and stretch the skin to facilitate needle insertion.
• High restraint: Gavage requires firm restraint to prevent the mouse from moving its head.
• Restraint tube: Injections into the caudal vein use restraint tubes that block the mouse’s movement while providing access to the tail without the risk of unexpected movement.
• Sedation or general anesthesia: Some administrations require working on a sedated or anesthetized animal due to the precision of the procedures, such as intradermal, intraosseous, or intrarectal administrations.
Figure 1: Examples of types of restraint and associated routes of administration.
The greater the restriction, the higher the stress felt by the animal.
Risks of error based on the route of administration:
The routes of administration vary in terms of danger to the animal. If a procedure is performed incorrectly, it can have scientifically significant consequences and/or important ethical consequences (see Table 2 and Table 3). We chose to distinguish between ethical and scientific consequences, although these two concepts are closely linked. Indeed, every ethical consequence leads to a scientific impact due to the physiological effects of pain and stress, as well as in cases of ethical euthanasia of animals, which result in a reduced sample size. The risk of performing this action incorrectly also depends on the procedures and can be reduced by using general anesthesia.
Table 2: Non-exhaustive list of routes of administration, the risk of error, and the scientific and ethical consequences in case of error; the sources used include the NC3R, the FC3R, Chapter 32 of the book The Laboratory Mouse » (Hedrich, 2004) and (Morton et al., 2001; Turner et al., 2011).
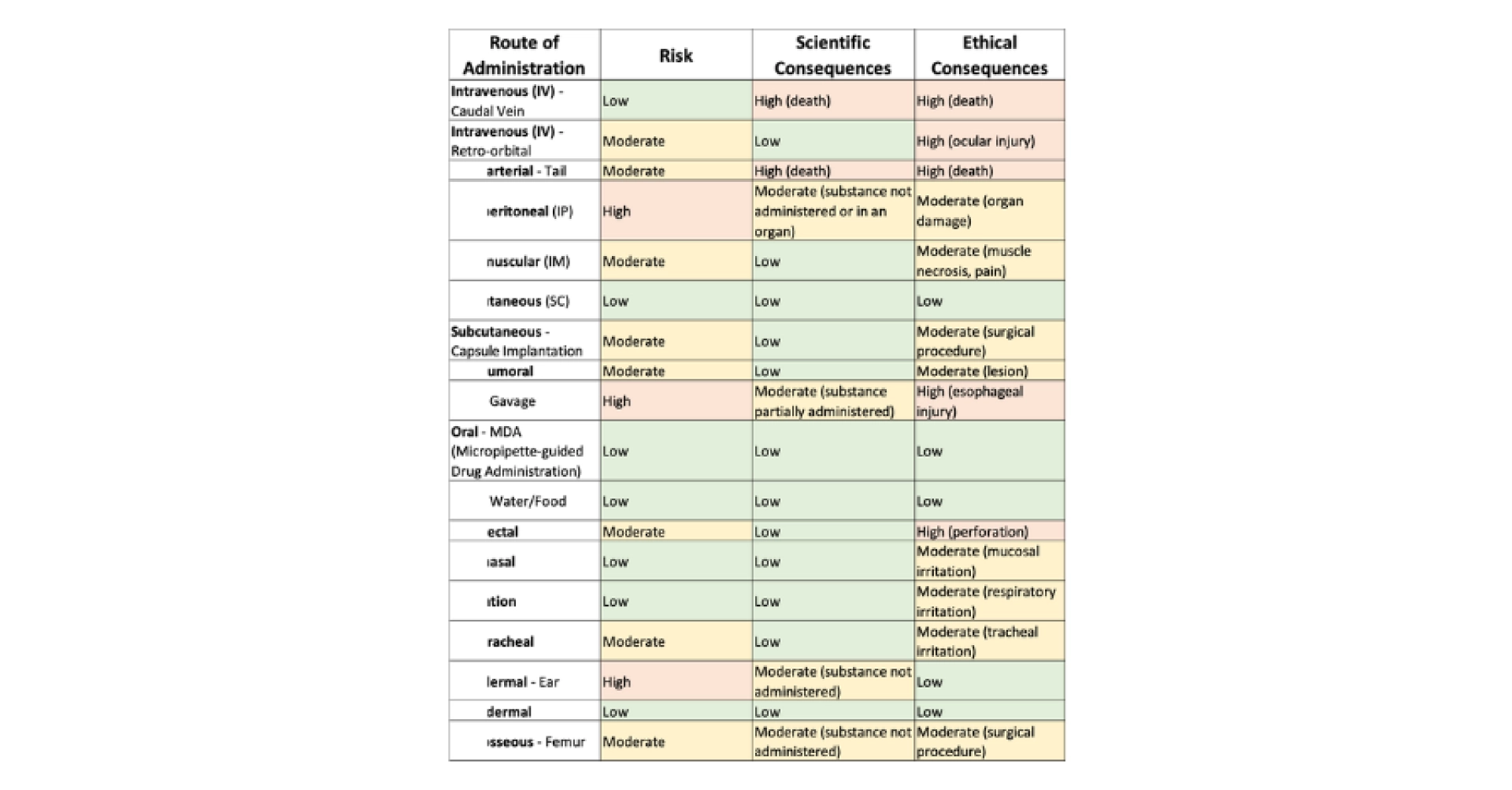
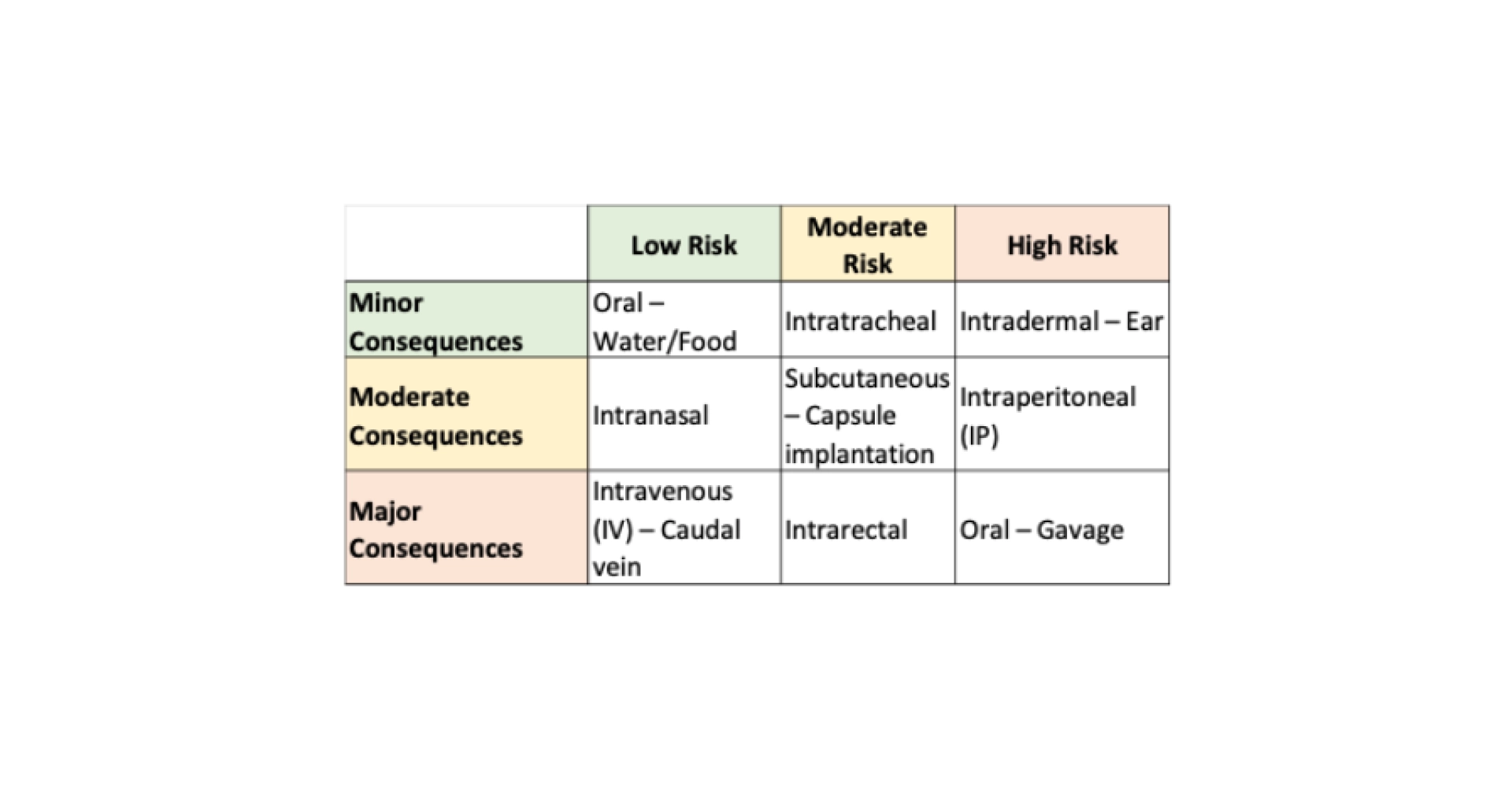
Table 3: Examples of administration methods based on the risk of error and the significance of the consequences (scientific and ethical) of an error.
The use of general anesthesia can help minimize the risk of error for certain administrations that may be dangerous for the animal. For example, retro-orbital administration can be performed on either a conscious or anesthetized animal, but the risk of eye damage is reduced when anesthesia is used.
Changing needles regularly:
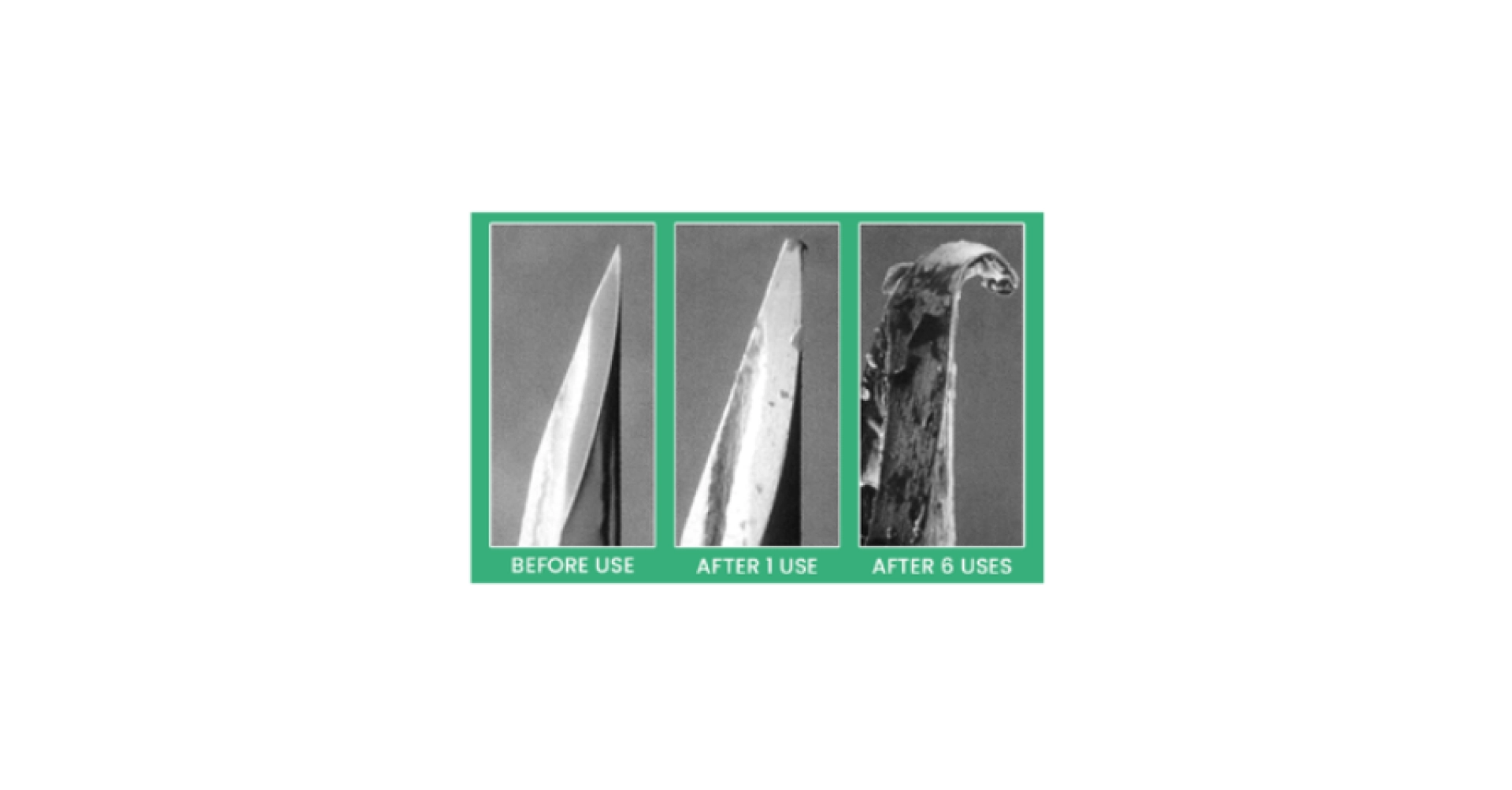
Many routes of administration use needles. When there is no risk of infection transmission between animals, needles are not always changed between each animal. However, it is recommended to change them as often as possible to minimize their deterioration and reduce the impact on animals (see Figure 2).
Figure 2: Illustration of needle degradation after multiple uses, from Injecting Step-by-Step – Resources for Safer Injection and Substance Use.
The well-being of technicians:
Certain administration methods can be daunting, particularly retro-orbital injection, which require inserting a needle behind the eye into the retro-orbital sinus. This method is effective, quick, and can be performed with or without anesthesia; however, the eye can be damaged if an error occurs. This method is increasingly criticized, used less frequently, and in some cases, even prohibited (Whittaker & Barker, 2020).
Repeated administration:
For certain research protocols, administrations need to be performed through the same route daily, sometimes multiple times a day. In such cases, the choice of administration method becomes even more critical. Gavage and injection into the caudal vein, in particular, are methods that cause harm to the animals, and the scientific and ethical consequences can be significant. Alternative methods can be selected: the MDA method to replace gavage, and the vascular access port (a close system to connect an implanted catheter to a syringe for direct access, or to a tether for continuous access) to replace repeated injections into the caudal vein.
Figure 3: Vascular acces port, image from Guide to Vascular Access Buttons™.

Needle size:
The thinnest needles should be selected to avoid fluid leakage and minimize discomfort during the injection. A viscous product or one containing cellular product requires the use of a larger needle diameter. The recommended needle sizes are available in Table 3 of Morton et al., 2001. The needle length should be adapted to the depth of the injection (Hedrich, 2004; Morton et al., 2001).
For gavage, flexible needles or needles with a spherical tip (made of metal or plastic) can be used, each with its own advantages and disadvantages. The length should be appropriate for the animal’s esophagus size to minimize the risk of perforation (Turner et al., 2011).
Numerous routes of administration can be used in mice. The selection of the most suitable route should be based on scientific objectives, ethical consequences, and consider factors such as the type of substance, formulation, volume, viscosity, and the need for repeated administrations. The choice of administration route can have a significant impact on the course of a study. Scientifically with varying distribution rates and potential hepatic passage. Ethically with risks to the animal that can vary in severity, as well as variable pain and stress levels.
New methods are being developed, and imaging allows us to observe the disadvantages of certain administration routes. For example, the MDA method can replace gavage (which is stressful, potentially painful, and risky) while the IP route is criticized for its risk of undetected errors (injections may occur in the intestine, stomach, bladder, muscle, or another organ without detection) and because it is not used for administrations in veterinary and human clinics (Al Shoyaib et al., 2020; Morton et al., 2001).